|
|
We can think of electromagnetic radiation in several different ways:
- From a physical science standpoint, all electromagnetic radiation can
be thought of as originating from the motions of atomic particles. Gamma-rays occur when atomic nuclei are split or fused. X-rays occur when an
electron orbiting close to an atomic nucleus is pushed outward with such
force that it escapes the atom; ultraviolet, when an electron is jolted
from a near to a far orbit; and visible and infrared, when electrons are
jolted a few orbits out. Photons in these three energy ranges (X-ray, UV,
and optical) are emitted as one of the outer shell electrons loses enough
energy to fall down to the replace the electron missing from the inner
shell. Radio waves are generated by any electron movement; even the stream
of electrons (electric current) in a common household wire creates radio
waves ...albeit with wavelengths of hundreds of kilometers and very weak
in amplitude.
- Electromagnetic radiation can be described in terms of a stream of
photons (massless packets of energy), each traveling in a wave-like
pattern, moving at the speed of light. The only difference between radio
waves, visible light, and gamma-rays is the amount of energy in the
photons. Radio waves have photons with low energies, microwaves have a
little more energy than radio waves, infrared has still more, then
visible, ultraviolet, X-rays, and gamma-rays. By the equation
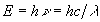 , energy dictates a
photon's wavelength and frequency. , energy dictates a
photon's wavelength and frequency.


 Download a pdf version.
Download a pdf version.
|
