|
Perhaps the most intriguing advance in
X-ray astronomy
instrumentation in the 1990s has been the development of single photon
calorimeters, spearheaded by work at NASA's Goddard Space Flight Center.
These devices detect X-rays by the temperature pulses they generate in a small
absorber which is cooled to a fraction of a
Kelvin.
When an X-ray stops in a detector, it gives all of its energy
to one electron. That electron can rattle around in the detector and
give energy to other electrons. All these excited electrons
would rather go back to their original energy. They want to return
to what is called the 'ground state'. Through scattering with other
electrons or with vibrations in the solid itself, they can lose that
extra energy. But that energy has to go somewhere. What it does
is heat the solid and increase its temperature. If you measure the change
in temperature, you can measure how much energy the X-ray originally
had.
How are heat and energy and temperature all related? Heat is a
manifestation of energy. Heat and energy are measured in the same
units (Joules). We usually think of energy as heat if we are thinking
of the total energy of a large ensemble of objects that can exchange
energy with each other and come into equilibrium. So, when an
X-ray photon heats a solid, it gives its energy to the whole solid.
On average, each atom is vibrating a little bit more than before the
X-ray hit. Temperature is the way we relate the total energy of
a system to its state of disorder (entropy). A physical property
called "heat capacity" tells us how much the temperature rises in
a material if we put in a certain amount of energy.
Suppose we wanted to measure the temperature increase due to an
X-ray photon being absorbed. We'd want a very sensitive thermometer,
something that had some physical property that changed a lot for
a small change in temperature. And we'd want the detector to have
a small heat capacity, so its temperature would change a lot for
a small change in energy. And you'd want to do the whole thing
at very low temperatures, because at room temperature there would
already be too much thermal energy in your detector to see the
very small change in energy from the X-ray. That is what an X-ray
calorimeter does. It uses a silicon thermistor which has an electrical
resistance which changes dramatically with small changes in temperature.
It has a low heat capacity. And it operates at less than 0.1 K.
That's Kelvin. Zero on the Kelvin scale is an absolute zero and
represents the cessation of all thermal vibrations. Water freezes
at 273 K. Nitrogen liquefies at 77 K. Helium liquefies at 4 K.
and we operate calorimeters at less than 0.1 K! Calorimeters
are able to get the best spectral resolution of any non-dispersive
spectrometer. |
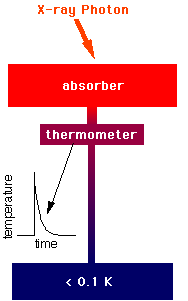
Diagram showing the
essential elements of
a calorimeter. Inset shows
temperature response to an
X-ray hit. |
