 |
|||
|
Early
Earth was a hostile environment unsuitable for human life.
|
|||
| THE EVOLUTION OF THE EARTH | |||
| The Earth started to form, along with the other planets, from material rotating around the young Sun about 4 thousand million years ago. Solid material came together in a process called planetary accretion. Heating caused changes within the planet, leading to differentiation into layers of different chemical and physical composition - the core, mantle and crust. This differentiation of the planet's interior is indirectly responsible for the Earth's atmosphere, oceans, continents, mountains and magnetic field. | |||
| Accretion | |||
| During the process of planetary accretion, small solid objects are drawn together by gravity, producing a larger object. The impact of objects on the proto-planet results in a conversion of the objects' kinetic energy into thermal energy - the planet heats up. Gravitational attraction also causes the planet to warm by compressing the material - converting gravitational potential energy into heat. As a result, a newly-formed planet, such as the Earth about 4.7 thousand million years ago, may reach a stable temperature of about 1000oC. | |||
| Given enough time, another source of heat comes into play and starts to dominate the proceedings. The heavy elements uranium and thorium, and the heavy isotope of potassium, spontaneously disintegrate into lighter elements, releasing subatomic particles (helium nucleii and electrons) in the process. This natural radioactive decay warms the surrounding material by transforming the kinetic energy of the released particles into heat energy. | |||
| Formation of the core | |||
| After maybe a thousand million years of warming by radioactive decay, it is estimated that a temperature of 1800oC would be reached at a depth of 500 kilometres - hot enough to melt iron. Because iron is heavier than most other material in the Earth, drops of the molten iron began to sink, displacing lighter material below. Because iron is so abundant, the melting process produced dramatic changes and this stage in the Earth's history is known as "the iron catastrophe". | |||
| About a third of the Earth's mass pooled at the centre of the planet to form a liquid core. The sinking of this amount of material created more heat by the release of gravitational energy. The average temperature of the planet increased by another 2000oC and a large fraction of the Earth's remaining solid material melted. | |||
| Core formation was the beginning of the process of differentiation. With much of the planet's mass in a molten, mobile state, lighter material was able to float to the surface and denser material to sink. Material reaching the surface rapidly cooled, losing it's heat to space, and any ocean of molten rock would quickly have frozen to form a solid crust of relatively light rock. The earliest surface rocks we can now find probably date from this time - about 4 thousand million years ago. | |||
| Convection | |||
| Loss of heat by conduction to the surface of a planet is a very slow process, but with much of the planet's interior in a molten state, convection was able to occur. Hot gas or liquid expands, becoming lighter in the process and rising above cooler material, carrying heat with it. Convection allowed much of the heat in the mantle, between the core and the crust, to be dissipated and the temperature to drop to a point where the rock solidified. Most of the iron core, however, remains in a molten state to this day. | |||
| Although convection is most common in liquids and gasses, it can occur in solids, particularly when they are weakened by extreme pressure. Convection continues in the mantle and is proposed as the driving force behind the large-scale geological processes on Earth today - seafloor spreading and the movement of the tectonic plates of the Earth's crust. | |||
| Convection provided a mechanism for the chemical zonation of the Earth's interior, with elements able to move vertically according to the properties of the compounds they formed with other elements. Chemical zoning has meant that heavy elements such as iron and uranium are found in the crust as silicates and oxides. Indeed, uranium and thorium have been concentrated in the crust, and this has slowed the internal heating of the Earth, since the heat from their radioactive decay is more easily dissipated from the crust than from the interior. | |||
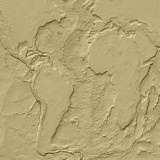 The Earth without oceans or vegetation. The shapes of the continents are still recognisable. |
|||
| Continents, oceans and atmosphere | |||
| It is expected that the early crust underwent weathering, erosion and sedimentation as happens today, with great thickness of lighter material building up in some places. This horizontal differentiation of the crust into thick, less dense areas and thin, dense areas has left the Earth's surface form dominated by the continents and ocean basins. It is reckoned that the growth of areas of continental crust was complete by about 2.5 thousand million years ago. | |||
| In addition to the solid crust, heating and differentiation also released water and gases from the interior. Hot clouds of water vapour would have condensed to liquid form and pooled at lower elevations on the surface. From the mixture of gases released by volcanic eruptions today, we can surmise that the early atmosphere was rich in water vapour, hydrogen, hydrogen chlorides, carbon monoxide, carbon dioxide and nitrogen. | |||
| Lightweight hydrogen molecules would have escaped into space as they do today. Some water vapour would have broken down in sunlight to hydrogen and oxygen, but free oxygen would have quickly combined with other gases and with metals in the crust to form metal oxides. Some of the carbon dioxide would have been removed from the atmosphere and fixed in the crust by chemical combinations with calcium, hydrogen and oxygen. | |||
| However, the chemical composition of Earth's atmosphere is very different to that expected from the outgassing of a differentiated planetary body. Outgassing of volatile components from interior rocks is accepted as the source of the Earth's atmosphere, and this would produce a mixture of water vapour, hydrogen, hydrogen chlorides, carbon monoxide, carbon dioxide and nitrogen. We can look to the atmospheres of Venus and Mars, our nearest planetary neighbours, for a hint of this early atmosphere : 95% carbon dioxide with very little or no oxygen. | |||
| If the processes applied to the evolution of Earth's atmosphere were much the same as those operating on Mars and Venus, why is Earth's atmosphere so different ? In other words, where has all the carbon dioxide gone? | |||
| The transformation from the early primitive atmosphere, which we would find poisonous, to the oxygen-rich atmosphere we enjoy today, is largely due to the development of life. | |||
| There are two mechanisms which changed the composition of the Earth's atmosphere, one of them biological and one of them geo-chemical, but still involving lifeforms. The most significant process is the removal of carbon dioxide from the atmosphere in water solution. A weak acid solution raining over exposed rock and soil causes chemical weathering, releasing various minerals as debris and solution into streams and rivers. The atmospheric carbon dioxide is now bound up in the mineral debris and carried to the ocean, where marine creatures use the dissolved minerals to precipitate shells of calcium carbonate. When they die, their shells sink to the seabed and form carbonate rocks - limestone. Estimates of the amount of carbon dioxide fixed in the rocks of the Earth's lithosphere roughly equal the amount estimated to be in the atmosphere of Venus | |||
| The process which produced the Earth's oxygen also removes carbon dioxide from the atmosphere, though mainly on a temporary basis. Photosynthesis is the conversion of carbon dioxide and water to carbohydrates and oxygen in the presence of sunlight. This process, occurring in all green plants from algae up, simultaneously releases oxygen and fixes carbon into organic matter - lifeforms. Oxygen began accumulating in the atmosphere when the amount produced by photosynthesis exceeded that consumed by chemical combination with the Earth's other gases and metals. | |||
| Most of the carbon dioxide trapped by photosynthesis is released when a lifeform dies and decays, but if the lifeform is buried, the carbon is fixed over the long term in sediments and then sedimentary rocks, large concentrations resulting in coal, oil and natural gas deposits. | |||
|
|
|||